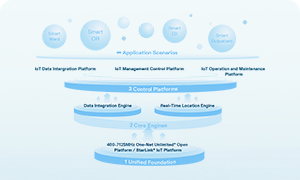
Global mainstream regulatory agencies and international standards have mandatory requirements for cold chain temperature monitoring. FDA, EMA, and other organizations require medical institutions to establish a comprehensive temperature recording system. PIC/S guidelines specify standardized requirements for cold chain equipment calibration, data integrity, and personnel training. ISO 13485 includes temperature monitoring as a core indicator of medical quality management systems.
A comprehensive temperature monitoring system can ensure that hospitals pass GSP certification, international medical quality reviews, and avoid compliance risks such as fines and business suspensions, while also meeting international requirements for data retention beyond the expiration date of drugs for at least one year.
CN
-
Solutions
-
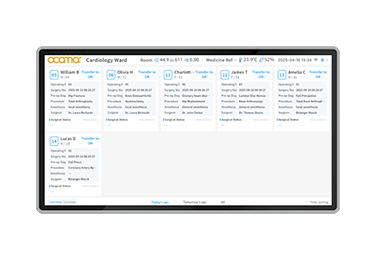
 RTLSInfant Security System
RTLSInfant Security System -
 RTLSWander Management System
RTLSWander Management System -
 Nurse Call System
Nurse Call System -
 Infusion Monitoring System
Infusion Monitoring System -
 Vital Sign Monitoring System
Vital Sign Monitoring System -
 Hospital Environmental Monitoring System
Hospital Environmental Monitoring System -
 RTLSAsset Management System
RTLSAsset Management System -
 Healthcare Mobile Computer
Healthcare Mobile Computer -
 Staff Duress System
Staff Duress System -
 Hand Hygiene Monitoring System
Hand Hygiene Monitoring System -
 Hospital Temperature Monitoring System
Hospital Temperature Monitoring System -
 Body Temperature Monitoring System
Body Temperature Monitoring System
-
-
Products
-
 RTLSInfant Tag
RTLSInfant Tag -
 RTLSMother Tag
RTLSMother Tag -
 Infant Band
Infant Band -
 RTLSAsset Tracking Tag
RTLSAsset Tracking Tag -
 Energy Monitoring Tag
Energy Monitoring Tag -
 Equipment Status Tag
Equipment Status Tag -
 RTLSWanderManage Wristband
RTLSWanderManage Wristband -
 RTLSWanderManage Wristband (Tamper-Resistant)
RTLSWanderManage Wristband (Tamper-Resistant) -
 RTLSStaff Badge
RTLSStaff Badge -
 IV Monitoring Sensor
IV Monitoring Sensor -
 Vital Sign Monitoring Mattress
Vital Sign Monitoring Mattress -
 Temperature Tag
Temperature Tag -
 Temperature Tag Patch
Temperature Tag Patch -
 Hospital Environmental Monitoring System
Hospital Environmental Monitoring System -
 Hand Hygiene Monitor
Hand Hygiene Monitor
-
- Resources
- About Us
- Partnerships